Suspension-feeding organisms (organisms that feed via removal of food particles suspended in the overlying water column), such as clams, mussels, oysters, and other invertebrates (e.g., brachiopods, tunicates, sponges) can remove substantial amounts of suspended particulates (‘seston’) from the overlying water column through their significant feeding activities. Water filtered by oysters contains a variety of particles of differing food value, including phytoplankton, particulate organic matter, and inorganic matter. Water column concentrations of chlorophyll a are often used as a surrogate for food ‘quantity’ for filter-feeding organisms. Use of high-performance liquid chromatography (HPLC) on water or sediment-derived samples can also shed light on the taxonomic groups making up the phytoplankton community using the presence or absence of diagnostic pigments profiles. This adds information on food ‘quality’ and related food size. Particulate organic matter, another potential food resource, can be estimated by ashing and getting dry weights and AFDW of filtered particles from water samples. Elemental analysis (stable isotopes or TOC analyses) can be used to estimate quantities of nitrogen and carbon in the particulate organic matter. Food supply (i.e., food flux) has two components: (1) instantaneous food concentration in the water, and (2) movement of the water in the vicinity of the habitat (see flow section).
The most commonly used methods for measuring seston removal from the water column (= uptake) involves taking discrete water samples collected either by dipping surface water, using a vertical or horizontal water sampling bottle (see below left) at various depths or by pumping water through a fluorometer or collecting water samples followed by laboratory analyses using either a fluorometer (e.g., Turner Designs, generally for lower concentrations from small volumes of filtered water) or spectrophotometer after extraction with acetone (e.g., EPA method 445). Chlorophyll-containing phytoplankton or suspended phytomicrobenthos are then concentrated onto glass fiber filters onto either a GF⁄C or GF⁄F filter (see below right) at low vacuum. Pigments are extracted in 90% acetone and read after 2-24 hours.


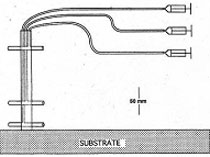
One simple and cost-effective approach uses a multiple syringe sampling device and associated current meter (see below two figures and Judge et al. 1993).

A newer method based on in situ fluorometry has recently been developed and evaluated (see below and Grizzle et al. 2006) providing a rapid measurement of seston removal rates in the field over various shallow habitats. The new system is comprised of two identical units, each consisting of an in situ fluorometer, data logger, and peristaltic pump(s) with plastic tubing attached to a deployment device. The deployment device allows precise placement of the fluorometer probe and intake end of the plastic tube so that in situ fluorescence (chlorophyll a) can be measured and water can be sampled for seston analyses in the laboratory from the same height. The typical setup involves placing one unit upstream and the other downstream of the study area and sampling the water at periodic intervals. Changes in seston concentration in real time may be directly read off the two fluorometer loggers, and concurrent water samples at one or more heights above the bottom can be analyzed later in the laboratory for various seston parameters (chlorophyll a, total seston, nutrients, salinity, etc.) using water collected into Whirlpack™ bags. Comparisons of the in situ near continuous data versus discrete data from laboratory analyses of pumped water samples can then be made. Concurrent flow sampling in 2 or 3-dimensions at one or more positions along the habitat of interest can also be conducted to provide food flux and other data for modeling.


Two in situ seston units deployed in the field (left) and single unit on the dock (right) (photos from R. Grizzle).
Judge, M.L., L.D. Coen, and K.L.Heck, Jr., 1993. Does Mercenaria mercenaria encounter elevated food levels in seagrass beds? Results from a novel technique to collect suspended food resources. Marine Ecology Prog. Ser. 92:141-150.
Grizzle, R.E., J.K. Greene, M.W. Luckenbach, and L.D. Coen, 2006. A new in situ method for measuring seston uptake by suspension-feeding bivalve mollusks. J. Shellfish Res. 25:643-649.